

The European Food Safety Authority (EFSA) recently released its scientific opinion on High Pressure Processing (HPP). The panels of experts involved in assessing the safety and efficacy of HPP reported that HPP effectively destroys pathogens such as Listeria monocytogenes, Salmonella, and E. coli, without posing no more food safety concerns than other commonly used treatments. In this scientific opinion, EFSA states that the HPP can be used to increase food safety in several products, such as ready-to-eat cooked meat products and milk. It was further concluded that HPP does not promote the migration from food contact materials into foods.
High Pressure Processing (HPP) has been used worldwide for more than two decades. Still, while the U.S. Food and Drug Administration (FDA) and Health Canada have established a regulatory frame for its use, the situation in Europe is not well defined, as HPP is not specifically regulated at the European Union level.

To address this issue, the European Commission requested the European Food Safety Authority (EFSA) to assess the efficacy and safety of HPP when used in food production. As a result, different aspects of the HPP technology were assessed, namely: i) the microbiological and chemical safety of HPP; ii) the efficacy of HPP applied to raw milk/colostrum; and iii) the efficacy of HPP to inactivate Listeria in ready-to-eat (RTE) foods. Consequently, EFSA recently published its scientific opinion on HPP.
Microbiological and chemical safety of HPP
It is the EFSA’s opinion that HPP of food does not present any additional microbial food safety concerns to consumers when compared to other common treatments that are routinely applied to foods. For instance, HPP is not expected to increase prion infectivity or induce the expression of genes related to virulence factors, toxin gene expression, and cross-resistance. Still, it is important to know that HPP does not inactivate spores. Consequently, EFSA points out the need to properly control the cold chain, maintaining the temperature below 7 °C during distribution and storage to prevent spore germination and outgrowth. It is noteworthy to mention that ESFA also considers that mycotoxins and process contaminants do not present an increased concern due to the use of HPP when compared to conventional food.
Potential chemical food safety concerns related to food contact materials (FCM)
In-Pack HPP is applied to pre-packaged products, thus flexibility and elasticity are the most important characteristics of HPP packaging. Thus, plastic packaging is the most suitable option, which raises concerns regarding the potential migration of chemicals into food-induced by HPP. In their report, EFSA concluded that there is no significant increase in migration from food contact materials to foods when compared to the same conditions without HPP. Indeed, in most studies, it is reported a decrease in migration/permeation during HPP compared to contact without HPP. Furthermore, it was also considered that the effect of HPP is insignificant in the possible formation of reaction products. Therefore, it was concluded that the migration testing performed with food simulants does not need to include the HPP step.
Efficacy of HPP applied to raw milk
There is an increasing interest in using HPP to process raw milk and thus obtaining a fresh and with increased shelf-life product.
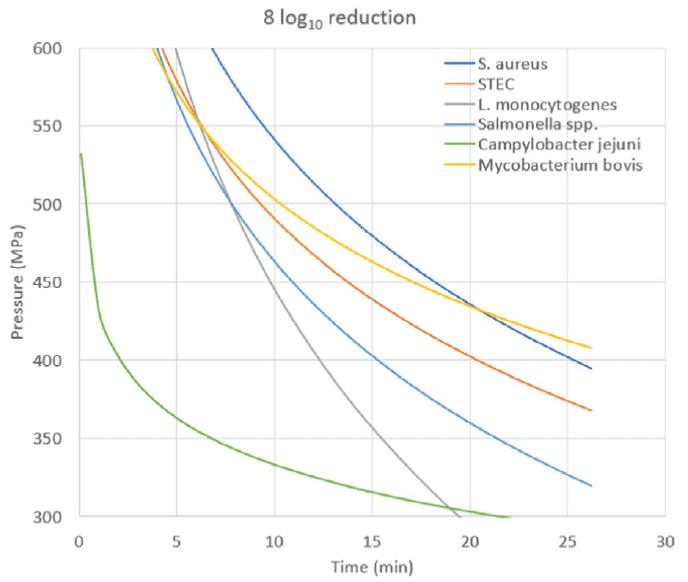
EFSA identified the most common HPP processing conditions used in the industry as 400-600 MPa for 1.5 – 6 min. This means that most commercial HPP applications fall within this range, however, it does not mean that different conditions cannot be used. Based on EFSA’s scientific opinion, HPP (600 MPa; 6 min), can achieve the performance criteria for several pathogens (M. bovis, E. coli, L. monocytogenes, Salmonella spp., and Campylobacter spp.), similarly to pasteurization. However, using these conditions, HPP may not achieve the performance criteria for S. aureus, resulting in a lower inactivation than that achieved by pasteurization. Still, increasing HPP time to 8 min (Figure 1) allows to also achieve the performance criteria for S. aureus (8 log10 reduction), similarly to pasteurization.
Indicators to verify the efficacy of HPP
The EFSA scientific panel determined that none of the evaluated indicators can currently be proposed as an appropriate indicator to be used under the technologically and commercially feasible HPP conditions applied by the industry, including alkaline phosphatase.
It is noteworthy to mention that alkaline phosphatase is commonly used as an indicator of a successful pasteurization of milk, given that is destroyed at a temperature near to the pasteurization temperature (similarly to the pertinent pathogens). This enzyme and pathogens in milk have a different resistance to pressure, which means that this enzyme cannot be used as an indicator of successful processing. However, the fact that it cannot be used as an indicator has nothing to do with the effectiveness of HPP on pathogens.
Efficacy of HPP to inactivate Listeria in RTE-cooked meats
HPP is commonly used as a post-lethality treatment to control Listeria monocytogenes in RTE-cooked meats. According to EFSA’s scientific opinion, HPP effectively controls this pathogen in RTE-cooked meats, allowing it to meet the requirements and/or recommendations of food safety agencies. For instance, EFSA predicts that applying 600 MPa for 4.7 minutes achieves a >5 log10 reduction of L. monocytogenes – Figure 2.
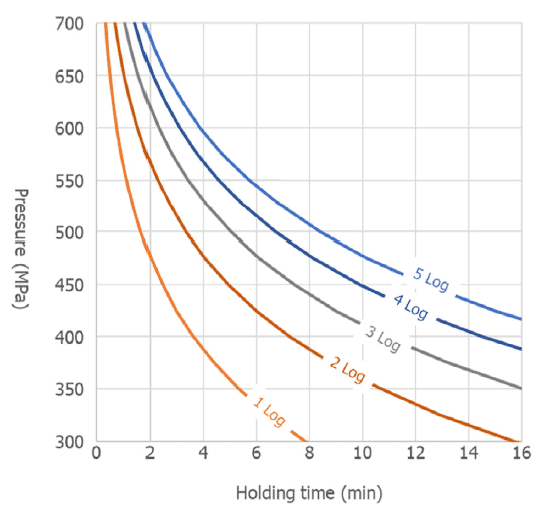
Salmonella spp. and pathogenic E. coli were identified as additional relevant hazards in this type of food. EFSA concluded that in RTE-cooked meats, these pathogens are generally more sensitive to pressure than L. monocytogenes and, thus, they will be inactivated to a similar or higher extent than L. monocytogenes. Still, further research is necessary on the pressure inactivation of L. monocytogenes and other relevant pathogenic bacteria for other RTE foods which could facilitate the construction of suitable predictive models to set the generic minimum requirements for HPP to assure the food safety of these food products. Therefore, for other types of RTE foods, specific validation studies (e.g. challenge tests) would be needed to set the HPP conditions required to achieve a given target log10 reduction.
Overall, EFSA concludes that HPP effectively destroys pathogenic bacteria and the process itself poses no more dangers to food safety than other commonly used treatment methods.
Feel free to contact us if you want to know more about HPP and its different applications.










