Hiperbaric, in collaboration with the University of Burgos – Spain, conducted a comprehensive assessment on the effects of High Pressure Processing (HPP) on cold brew coffee. The outcome revealed that HPP significantly inactivated pathogenic bacteria, achieving a notable 6-log reduction and ensuring safety throughout a substantial 90-day period. Noteworthy was the preservation of the characteristics of the beverage, including color and overall freshness, underscoring the potential of HPP to maintain the quality of cold brew coffee. This research contributes pertinent insights for the production and processing of cold brew beverages.
HPP Cold Brew Coffee
Coffee, with its pleasant aroma, taste, and stimulating effects, is a widely appreciated beverage. Recently, cold brew coffee has emerged as a popular choice among consumers, offering a refreshing alternative to the traditional cup of hot coffee. Unlike regular hot-brewed coffee, cold brew is crafted by steeping coarse coffee grounds in cold water for an extended period, resulting in a smooth, less acidic flavor profile with a subtle caffeine boost. Its refreshing taste and artisanal profile make it a laid-back alternative to the usual coffee routine.
In response to high consumer demand, producers of cold brew coffee are exploring the suitability of High Pressure Processing (HPP) to ensure safety and extend the shelf life of this beverage. To address this trend, Hiperbaric partnered with the University of Burgos, Spain, to conduct a comprehensive assessment on the effects of HPP on cold brew coffee from both safety and quality perspectives.
In this study, cold brew coffee underwent processing at 6000 bar for 3 minutes, adhering to industry standards. The evaluation included the impact on inoculated pathogens (five-strain cocktails of Escherichia coli O157:H7, Listeria monocytogenes, and Salmonella enterica), microbial spoilage indicators, physicochemical quality parameters, and bioactive compounds. The findings of this investigation, titled “High Pressure Processing (HPP) for Cold Brew Coffee: Safety & Quality Assessment Under Refrigerated and Ambient Storage” have recently been published in the scientific journal Foods – Figure 1.

Figure 1 – Scientific publication of the results obtained in the study of HPP cold brew coffee.
HPP as a Kill Step to Ensure Safety
The initial concentration of the pathogens inoculated on the cold brew coffee samples ranged from 7.0 to 7.4 log CFU/ml. Pathogen growth was not observed on untreated cold brew coffee samples, yet all three species tested persisted for a minimum of 60 days at 4 °C. Over the initial 14 days, the load of E. coli O157:H7 and L. monocytogenes remained relatively constant, whereas S. enterica exhibited a reduction of 2 log CFU/ml. Subsequently, notable reductions in cell counts were observed, particularly in L. monocytogenes and S. enterica. However, a 5-log reduction was only evident after a prolonged 60-day storage period – Figure 2. The prolonged viability of these pathogens highlights the imperative for an effective kill step.
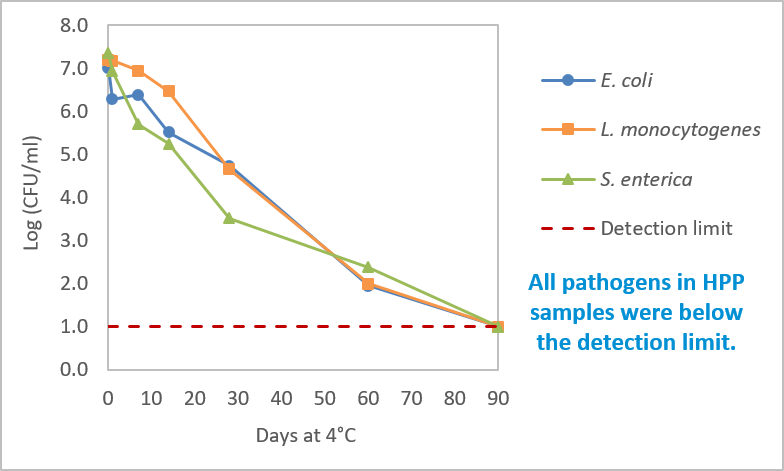
Figure 2 – Concentration of E. coli O157:H7, L. monocytogenes, and S. enterica on unprocessed cold brew coffee through 90 days of storage.
HPP resulted in the reduction of counts for all three pathogens to below the detection limit (1 log CFU/ml), surpassing a noteworthy 6-log reduction. This reduction was sustained for 90 days, highlighting HPP’s efficacy as a tool in ensuring the safety of cold brew coffee.
Quality Assessment
Cold brew coffee is generally a fairly stable product. This stability owes itself to the fact that cold brew coffee lacks essential nutrients for bacterial metabolism and has natural compounds with antimicrobial properties. The study found that the initial microbial load in unprocessed cold brew coffee was quite low, with less than 1.3 log CFU/ml of total aerobic bacteria. Importantly, all microbial indicators remained below this value over 90 days. This emphasizes the stability of high-pressure processed cold brew coffee against potential microbial issues.
Microbial Spoilage Indicators
High pressure processing (HPP) is a non-thermal food preservation technology that applies high hydrostatic pressure to food and beverage products in their final packaging, inactivating foodborne pathogens.
Physicochemical Quality Parameters
The physicochemical quality parameters of cold brew coffee play a crucial role in defining its characteristics. Total dissolved solids (TDS) serve as an indicator of the “body” of coffee, influencing its strength and overall flavor profile. Unprocessed cold brew coffee samples exhibited an initial TDS of 1.4%, a value unaffected by HPP. TDS remained fairly stable throughout storage. The pH and titratable acidity contribute to the perceived acidity in coffee brews. The initial pH of cold brew coffee samples was 5.9, in line with the typical range found in commercially available cold-brewed coffee. Over the 90-day storage period at 4 °C, both unprocessed and HPP-treated cold brew coffee displayed a similar slight decrease in pH. Titratable acidity, initially unaffected by HPP, increased at a comparable rate in both processed and unprocessed samples.
The color of cold brew coffee was unaffected by HPP immediately after processing, and remained stable for 60 days at 4 °C. After that, there was a minimal change in total color difference. Similarly to the other characteristics, bioactive and antioxidant compounds were generally unaffected by HPP – Figure 3.
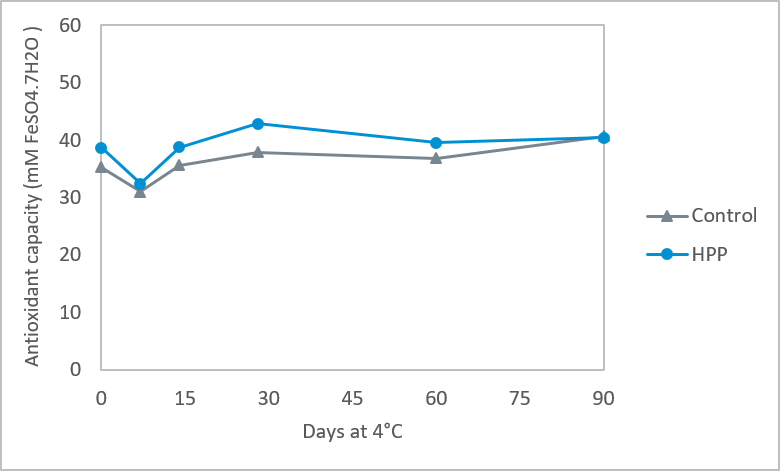
Figure 4 – Commercial examples of cold brew coffee and cold brew coffee-based beverages benefiting from HPP.
These findings underscore the minimal impact of HPP on the physicochemical parameters of cold brew coffee, highlighting its stability and maintaining key characteristics over an extended storage period.
Conclusions of the Research
The study revealed that HPP is an effective kill step, ensuring the safety of cold brew coffee by significantly inactivating pathogens. This reduction, exceeding a remarkable 6-log reduction, remained for an impressive 90-day storage period, which highlights the potential of HPP to ensure safety of this beverage. Beyond safety considerations, the assessment of the quality parameters of cold brew coffee demonstrated the versatility and reliability of HPP.
Notably, the nonthermal nature of HPP proved advantageous in preserving the color and overall freshness of cold brew coffee. The stability of cold brew coffee was further emphasized through minimal alterations in physicochemical parameters, even after extended storage periods. The findings collectively underscore the potential of HPP as an important tool in ensuring both, the safety and the quality of cold brew coffee.
Commercial Examples
In addition to this investigation, several commercial brands have autonomously embraced the advantages of HPP to extend the shelf life and enhance the safety of cold brew coffee and cold brew coffee-based beverages. Concurrently, these brands have successfully preserved the quality and sensory attributes of their products. Noteworthy examples of such cold brew coffee-based beverages are offered by brands such as Cafè Fred, Raw Pressery, Secret Squirrel Coffee, REBBL, and La Casa del Jugo – Figure 4.

Figure 4 – Commercial examples of cold brew coffee and cold brew coffee-based beverages benefiting from HPP.
Feel free to contact us if you want to know more about HPP applications for cold brew beverages and all the benefits that this technology can offer.