High Pressure Processing (HPP) has been commercialized for almost 30 years all over the world with no history of product outbreaks. This is attributed to the flexibility of the technology and to the fact that it can be easily integrated in Hazard Analysis and Critical Control Points (HACCP) plans. However, there are different applications for HPP and not always pathogen control is the main objective. How does HACCP work? How can Hiperbaric help your business integrating HPP? Keep reading to learn more details…
Food safety standards have never been higher. While shopping, consumers pay more attention to the nutritional profile of the salsa tub they hold in their hands than to the potential presence of pathogens in it. While filling the cart with a couple sliced deli meat packs, scenes of friends sitting around a table enjoying the meat instead of scary microscopy images of Escherichia coli cells cross through our minds (unless you are a microbiologist… I feel you!)

We assume that the food we purchase is safe. And most likely we are right. Positioning a product on a supermarket shelf means that the food has passed very stringent controls. Industry implementation of risk assessment tools has helped to achieve this goal. Did you know that NASA is behind this success story?
The theory of Hazard Analysis and Critical Control Points (HACCP)
Space travels enclose several risks. This is something NASA learnt in the 1960’s. To minimize them, the space agency developed a risk assessment management tool to identify specific hazards within a process, determine their significance and develop appropriate controls to ensure that they did not reach the astronauts. The landing on the moon of Apollo 11 proved that the system worked well.
Regulators and the food industry adopted the same scheme as they saw on HACCP principles an opportunity to improve food safety. The general idea is the implementation within a process of appropriate control measures for specific hazards and the associated risk to consumers. Together with Good Manufacturing Practices (GMP) and Prerequisite Programs (PRP), they constitute the Food Safety Management System of a food business operator (Figure 1).
Seven basic principles are used in the development of HACCP plans (Figure 1). If a deviation occurs indicating that control has been lost, the deviation is identified and appropriate steps are taken to reestablish control and assure that hazardous products do not reach the consumer.
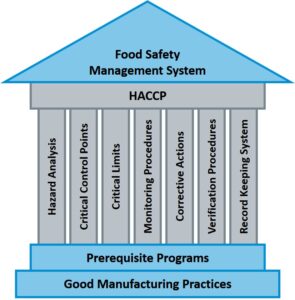
Hazard Analysis will identify potential hazards within a process that poses a risk to the final consumer. Adequate control measures must be implemented to address these hazards. When the implemented control is essential to prevent or eliminate a food safety hazard (or reduce it to an acceptable level) it is considered a Critical Control Point (CCP).
Addressing biological hazards with minimum impact on food quality
Biological hazards may lead to foodborne infections or intoxications. According to the World Health Organization, unsafe food causes 600 million cases of foodborne diseases and 420,000 deaths (WHO, 2015). To reduce or eliminate the risk associated to biological hazards, food preservation technologies are implemented. Traditional heat pasteurization has played a key role since the beginning of the 19th century in ensuring hygiene and protecting the health of the population.
Two centuries later, consumers demand minimally processed food with fresh-like properties that heat processing cannot achieve. Alternative methods such as nonthermal preservation technologies are gaining interest. High Pressure Processing (HPP) is by far the most widely implemented nonthermal technology worldwide. In addition to meeting the high-quality standards that consumers demand, HPP must reduce or eliminate biological hazards that the food may pose to fit into a HACCP plans. But is always HPP considered a Critical Control Point?
HPP as Critical Control Point (CCP) in HACCP plans
Food safety intervention
In-pack HPP is typically applied at the end of a production process on already packaged products, so no subsequent steps would eliminate or reduce biological hazards to an acceptable level. Therefore, HPP is considered a CCP in these cases. Critical Limits, Monitoring and Verification Procedures, Corrective Actions and Record Keeping Systems must be defined (Figure 2).

Hiperbaric’s Applications and Food Processing Department works closely with users of HPP technology to define Critical Limits to ensure pathogen control. Some food safety agencies (such as FDA in the US) require process validation to demonstrate that Critical Limits effectively achieve the required logarithmic reduction of pertinent pathogens. Hiperbaric has created an extensive HPP Academia Network that comprises universities, R&D and lab testing centers to assist the food industry with product validation and challenge studies.

Hiperbaric’s HPP equipment range incorporates Monitoring and Record Keeping Systems. Therefore, in the hypothetical case that Critical Limits are not reached, the HPP unit will hold the food products until Corrective Actions are taken. All of this information is continuously monitored and stored thanks to the SCADA system. Moreover, Hiperbaric also facilitates protocols to verify that measuring instruments (i.e. pressure transducers, chronometers and thermometers) operate properly.
Meat shucking from mollusks and crustaceans
Another important application of HPP technology is the extraction of meat from mollusks and crustaceans. In this case, seafood is processed in bulk (not packaged) and subjected to relatively low pressure levels (250-300 MPa/36,300-43,500 psi). Subsequently, meat from crustaceans and bivalves is easily extracted from the shells while keeping it raw. Then the meat pieces are packaged and ready for distribution.
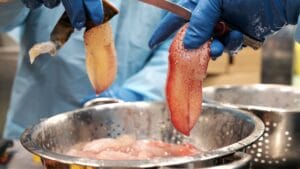
The low pressure levels used for this particular application are not sufficient to control pathogens such as Listeria monocytogenes. Hence, HPP cannot be considered a CCP for this hazard and additional controls are required to minimize the risk (freezing or reprocessing after packaging are common interventions).
Nonetheless, other pathogens frequently associated to seafood such as Vibrio spp. are pressure sensitive and can be controlled at low pressure levels. FDA considers HPP a suitable technology to control V. parahaemolyticus and V. vulnificus (Fish and Fishery Products Hazards and Controls Guidance, 2021). Special care must be taken handling the seafood after HPP to prevent cross contamination.
If you’d like to learn more about HPP and food safety, contact us!